How Surface Finishes Are Improved Through Electropolishing
Electropolishing removes metal in a highly ionic solution by means of electrical potential and current.
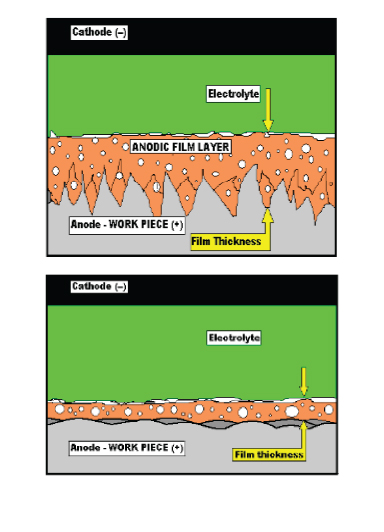
When the power is applied an anodic film forms on the surface of the work and the material begins to be removed ion by ion. The effect on the microscopic surface is to smooth and level as the microscopic “peaks” dissolve more rapidly than the microscopic “valleys” due to the increase in resistance to current flow as the film get thicker in the valleys.
When the process is allowed to continue for an adequate amount of time the surface becomes microscopically smooth and virtually featureless. As the anodic film becomes uniform in thickness the benefits of electropolishing have been accomplished and material will continue to be removed uniformly until the process is stopped.
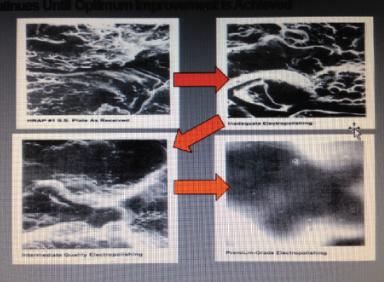
Proper electropolishing will not only provide a microscopic featureless surface, but will also reduce the total surface area as indicated in the samples below.
As electropolishing exposure increases, microscopic smoothing continues until optimum improvement is achieved as shown in the picture below.